Polyoxadiazoles (POD)
Properties
Polyoxadiazoles (POD) are a relative new class of high performance thermoplastic resins1 having oxadiazole groups and aromatic rings in their repeat unit. Despite their excellent thermo-mechanical properties, this class of polymers has gained only a modest market share among the engineering polymers. Their use is probably limited by their high price, and by difficulties in synthesis and processing.
Like other aromatic rigid polymers, fully aromatic polyoxadiazoles are insoluble in most organic solvents and exhbit very high glass transition temperatures and no melting point. To improve processing and solubility in solvents, bulky or noncoplanar groups such as naphthalene and/or groups with greater rotational freedom such as -O-, and -CH2- have been included in the polymer chain. The incorporation of bulky groups disrupts the crystal packing whereas groups with greater rotational freedom increases the flexibility of the polymer backbone. Both reduces intermolecular interactions and enhances solubility of the resulting polymer.
Polyoxadiazoles have a high decomposition temperature and exhibit outstanding thermal mechanical properties. Thus, they are well suited for high temperature applications. Other useful properties include high young's modulus (stiffness), outstanding abrasion resistance, low dielectric properties, high flame retardance, high resistance to organic solvents and good resistance to diluted inorganic acids and bases. However, (unmodified) polyoxadiazoles also exhibit some deficiencies including high curing temperature, low solubility, and low fracture toughness. These weaknesses can be (partially) overcome by including other monomers besides simple diacids in the polymer structure.
Synthesis
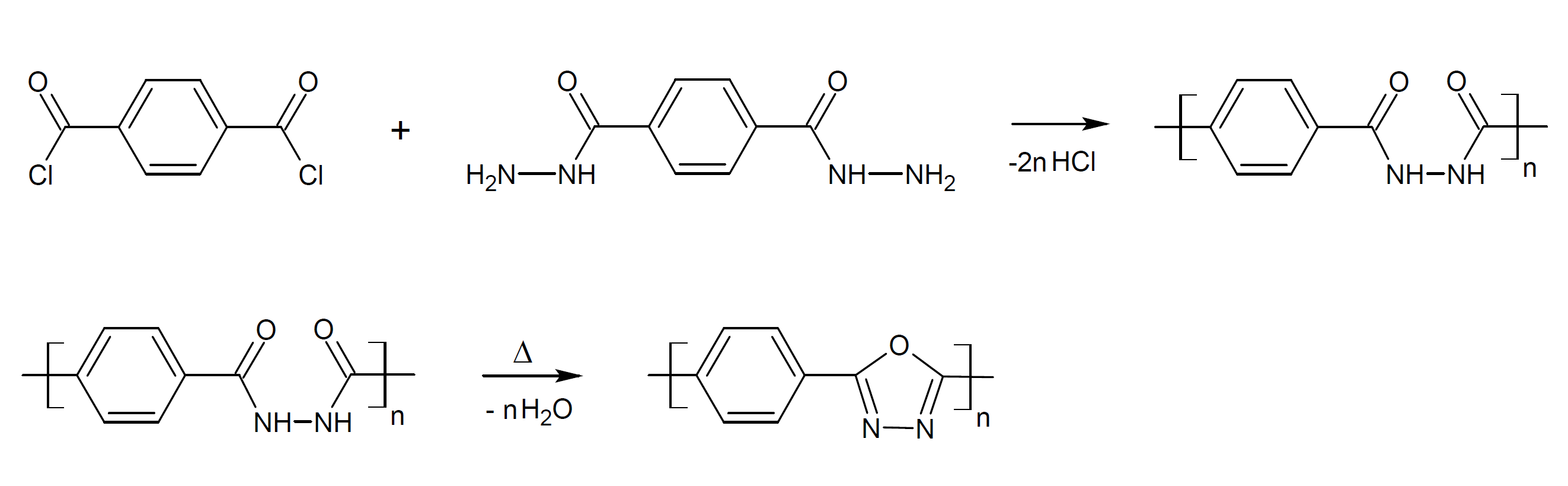
Polyoxadiazoles can be produced through the reaction of a dihydrazide with a diacid. The reaction involves a condensation and cyclodehydration step. The first reaction is typically carried out in solution at low temperatures and under argon atmosphere which produces a polyhydrazide. The washed and dried intermediate is then transformed to a polyoxadiazole by cyclodehydration at 160 to 200°C :2,3
Polyoxadiazole can also be produced via condensation polymerization of a dicarboxylic acid with a salt of hydrazine sulfate. The reaction in solution leads directly to the formation of polyoxadiazoles:
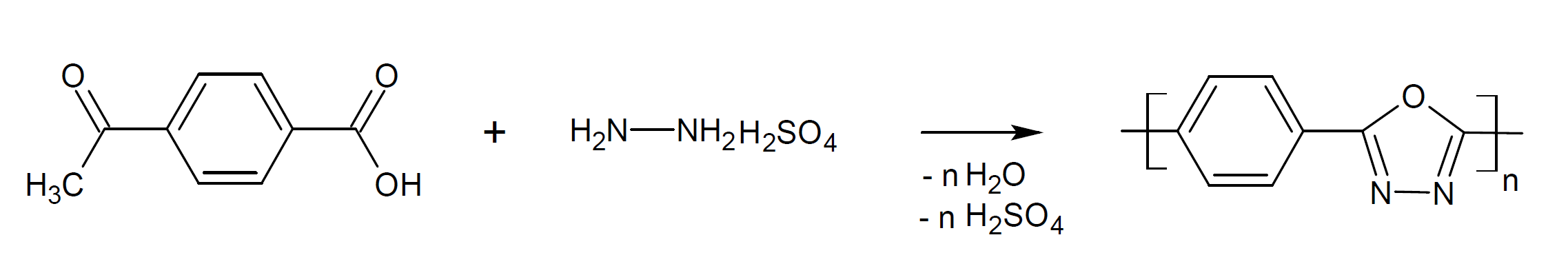
Depending on the reaction conditions, the method and the type of diacid or diacid chloride, some residual hydrazide groups can be present in the polymer, resulting in a poly(oxadiazole-hydrazide) copolymer.
COMMERCIAL Polyoxadiazoles
The only manufacturer of polyoxadiazoles is Khimvolokno Svetlogorsk which is located in the Republic of Belarus. The company produces polyoxadiazole fibers which they market under the registered trademark “Arselon”.
Applications
Polyoxadiazoles are mainly used for high performance textile products and as fiber reinforcements. Important applications include heat resistant garments like safety gloves and firerfighting uniforms; rubber and plastic reinforcements for hoses, tires and belts; heat and chemical resistant filters; friction pads, abrasion-resistant bearings and bushings, and various other civil engineering textile products. Polyoxadiazoles can also be converted to thin films to be used as membranes for gas separation or in fuel cells.2
The maximum service temperature of POD fibers is typically in the range of 230 - 250°С, and their thermal decomposition temperature is in the range 460 - 480°С.4
1Imai, Journal of Applied Polymer Science, Vol. 14, pp. 225-239 (1970); Patents: US 2008/0085992 A1, US 7528216 B2, DE 3620022 A1, DE 296277A5
2D. Gomesa, S.P. Nunesb, J.C. Pintoa, & C. Borges, Polymer, 44, 3633–3639 (2003)
3The intermediate can also be transformed into polyoxadiazole in solution using a dehydration agent.
4Y.N. Sazanov, I.P. Dobrovol’skaya, V.A. Lysenkob, P.Y. Sal’nikova, D.S. Kosyakov, S.A. Pokryshkin, G.N. Fedorova, and E.M.
Kulikova, Russian Journal of
Applied Chemistry, Vol. 88, 8, pp 1304–1310 (2015)